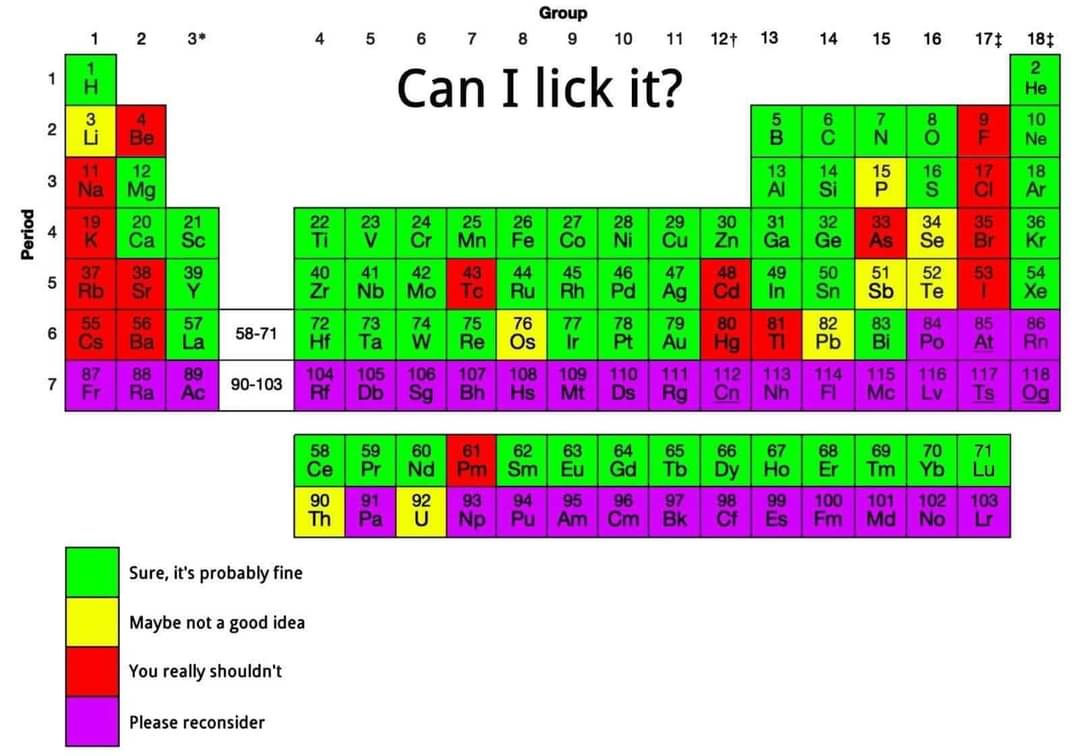
We can't lick sodium or chlorine, but combine them and you get something we literally make blocks of for the purpose of licking. What a world!
Science Memes
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- !abiogenesis@mander.xyz
- !animal-behavior@mander.xyz
- !anthropology@mander.xyz
- !arachnology@mander.xyz
- !balconygardening@slrpnk.net
- !biodiversity@mander.xyz
- !biology@mander.xyz
- !biophysics@mander.xyz
- !botany@mander.xyz
- !ecology@mander.xyz
- !entomology@mander.xyz
- !fermentation@mander.xyz
- !herpetology@mander.xyz
- !houseplants@mander.xyz
- !medicine@mander.xyz
- !microscopy@mander.xyz
- !mycology@mander.xyz
- !nudibranchs@mander.xyz
- !nutrition@mander.xyz
- !palaeoecology@mander.xyz
- !palaeontology@mander.xyz
- !photosynthesis@mander.xyz
- !plantid@mander.xyz
- !plants@mander.xyz
- !reptiles and amphibians@mander.xyz
Physical Sciences
- !astronomy@mander.xyz
- !chemistry@mander.xyz
- !earthscience@mander.xyz
- !geography@mander.xyz
- !geospatial@mander.xyz
- !nuclear@mander.xyz
- !physics@mander.xyz
- !quantum-computing@mander.xyz
- !spectroscopy@mander.xyz
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and sports-science@mander.xyz
- !gardening@mander.xyz
- !self sufficiency@mander.xyz
- !soilscience@slrpnk.net
- !terrariums@mander.xyz
- !timelapse@mander.xyz
Memes
Miscellaneous
One is bad in one way and the other is bad in the opposite way.
Neutralize!
But does this imply licking it in a "lickable" state? I have a hard time imagining licking a gas, and licking hydrogen as a liquid at -250 C or so sounds, not great.
Depending on the quantity and the leidenfrost effect, you might be fine
That's hilarious because me and my brother licked lead fishing weights for fun as a child. It's probably why I'm retarded.
Can someone make one for suitability as dildo material?
Edit: Here it is, chumps

Please don't lick elemental hydrogen.
Out of curiosity, what would happen if you do?
In the hypothetical, if one were able to lick elemental hydrogen in its atomic, rather than molecular form, it would have a few potential effects. The one that would concern me most would be its aggressive reactivity, ripping hydrogens away from anything that it could in order to achieve stability. This would potentially cause tissue damage both from the deprotonation and shift in pH.
Nothing, because you can have only one atom of it. Multiple will just form molecular hydrogen H2. That one hydrogen atom will aggressively rip of another hydrogen of a molecule of water for example, but it won't be noticeable.
Lick my As! You chemists can't stop me from slobbering on every element.
I'd say downgrade Mercury to yellow. Licking Mercury won't hurt you as long as you hold your breath.
Having it close to your breathy parts is always not a great idea though.
CodySlab swallowed it
My lead sandwich is calling to me
Mmm Pb&J.
I'm gonna lick Ununennium and you can't stop me
I always wanted to play with bromine. It looks so cool.
Why all the coolest things have to be toxic 😞 (broad life wisdom statement)
laughs in compound
You can't lick Titanium?
Titanium (22) is save to lick. Enjoy!
Ahh I saw Tl and mistake it as Ti XD
I had to look it up as well ;)