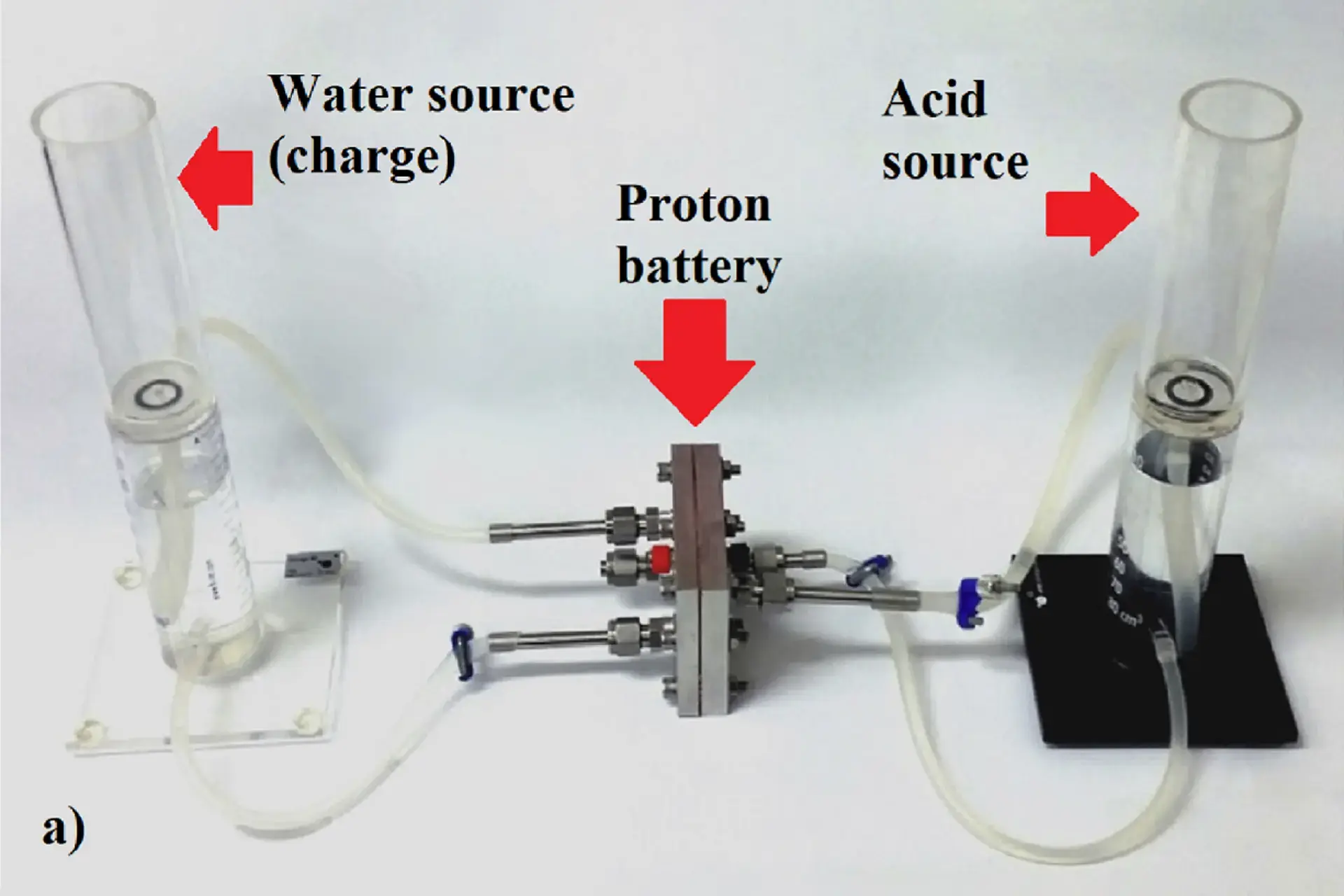
This is such a puzzle, thank you. :)
I checked Sci-Hub - no matches for "proton battery", neither for "hydrogen flow battery".
Falling back on chemistry - I recalled that "dissolving a concentrated acid in water should be done with care". It is exothermic, water may suddenly boil and splash acid all over the careless chemist.
By definition, acids are substances that can easily give protons (hydrogen ions) to other chemicals. A classic reaction would be acid + base = salt + water (acid gives the H and base gives the OH, so we get H2O), the other components of the acid and base form the salt.
If there is only water on the other side, I thought "the reaction is acid giving protons to water". Which acid? How many protons? Those questions might determine the amount of power available. And of course - how to control the reaction and extract electrical power? Browsing Wikipedia, I came across two pages: protonation and deprotonation and a sample reaction with sulphuric acid, but found no reference to electricity production, though potential / voltage is obviously available when ions are being created and transfered.
Then, finally I found the RMIT press release, and understood that acid is not a central participant of this reaction:
https://www.rmit.edu.au/news/all-news/2018/mar/all-power-to-the-proton
Some pickings:
The working prototype proton battery uses a carbon electrode as a hydrogen store, coupled with a reversible fuel cell to produce electricity.
It’s the carbon electrode plus protons from water that give the proton battery it’s environmental, energy and potential economic edge, says lead researcher Professor John Andrews.
During charging, the carbon in the electrode bonds with protons generated by splitting water with the help of electrons from the power supply. The protons are released again and pass back through the reversible fuel cell to form water with oxygen from air to generate power. Unlike fossil fuels, the carbon does not burn or cause emissions in the process.
The researchers’ experiments showed that their small proton battery, with an active inside surface area of only 5.5 square centimetres (smaller than a 20 cent coin), was already able to store as much energy per unit mass as commercially-available lithium ion batteries. This was before the battery had been optimised.
“Future work will now focus on further improving performance and energy density through use of atomically-thin layered carbon-based materials such as graphene, with the target of a proton battery that is truly competitive with lithium ion batteries firmly in sight,” Andrews said.
There is a photo of the three scientists with a cell and multimeter, and the meter reads 1.1559 volts, so we know the cell voltage is low, but not impractically low. A link to a scientific article and a description of the cell follows:
"Technical feasibility of a proton battery with an activated carbon electrode"
The latest version combines a carbon electrode for solid-state storage of hydrogen with a reversible fuel cell to provide an integrated rechargeable unit.
During charging, protons produced by water splitting in a reversible fuel cell are conducted through the cell membrane and directly bond with the storage material with the aid of electrons supplied by the applied voltage, without forming hydrogen gas.
(this implies that overcharging results in hydrogen formation, like in lead acid batteries - the solution is to have it vented typically)
In electricity supply mode this process is reversed; hydrogen atoms are released from the storage and lose an electron to become protons once again. These protons then pass back through the cell membrane where they combine with oxygen and electrons from the external circuit to re-form water.
(this leads to the question of oxygen availability on the other side, and how to ensure it's adequate - gases are a nuisance due to their low density, but water can dissolve only so much oxygen, and this could limit the power output or storage capacity of the cell, however, if one built a flow battery, a redundantly large mass of water could be used to supply oxygen - but I'd really like to know if they used gaseous or dissolved oxygen)
Therefore, in the proton battery, many processes in the conventional hydrogen-based energy storage system that cause energy losses and irreversible entropy increases are omitted, such as hydrogen gas evolution and compression, and the splitting of molecular hydrogen into protons in fuel cell mode.
A summary of the scientific paper:
The experimental results reported here show that a small proton battery (active area 5.5 cm2) with a porous activated carbon electrode made from phenolic resin and 10 wt% PTFE binder was able to store in electrolysis (charge) mode very nearly 1 wt% hydrogen, and release on discharge 0.8 wt% in fuel cell (electricity supply) mode. A significant design innovation is the use of a small volume of liquid acid within the porous electrode to conduct protons (as hydronium) to and from the nafion membrane of the reversible cell. Hydrogen gas evolution during charging of the activated carbon electrode was found to be very low until a voltage of around 1.8 V was reached. Future work is being directed towards increasing current densities during charging and discharging, multiple cycle testing, and gaining an improved understanding of the reactions between hydronium and carbon surfaces.
So, the acid was not a reaction participant, but a proton conductor.
If anyone has a copy of the paper, please share - it seems like it would be interesting. :)